5 Minute Healthtech Jargon Buster: Functional Imaging
- Romilly Life Sciences

- Apr 9, 2025
- 7 min read
by Lillian Hall, Research and Communications Associate
Functional imaging is a powerful non-invasive technique that provides an insight into the mechanisms of brain activity, achieved by tracking alterations in blood flow and oxygenation. Cortical areas which have higher blood flow are detected and used to infer brain activity (Stefano et al.). This helps researchers and medical professionals, such as psychologists and neurologists, to understand how the brain functions, how diseases affect it, and how it recovers from injury.
Several steps must be undertaken prior to the data being used for research or medical purposes. The first step, called preprocessing, transforms raw scanner data into clear brain images. Since the scan captures different slices of the brain at slightly different times, slice-timing correction adjusts them, so they appear synchronised (Smith).

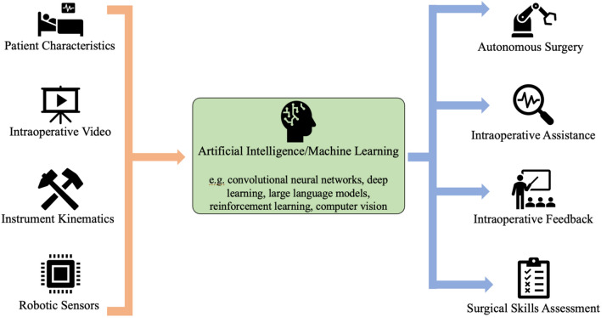
Figure 1 Segmentation of a foetal MRI scan using AI. (Lo et al.)
Next, motion correction ensures that all brain images align properly, even if the subject moved slightly during the scan. To reduce noise and improve clarity, researchers apply spatial smoothing, which slightly blurs the images without losing important details. Finally, intensity normalization adjusts brightness levels across all images so that the data is consistent. (Smith)
As Artificial intelligence (AI) continues to advance, it is playing an increasingly important role in automating and improving these processes, making functional imaging more efficient and insightful than ever before. The uses and challenges of its applications are detailed below.
Types of AI in Functional Imaging
AI is transforming the field of functional imaging by enhancing the ability to interpret and analyse complex data from imaging. The use of machine learning (ML) and deep learning (DL) algorithms have enabled this, which are described below.
Machine Learning
Machine learning is a form of artificial intelligence that focuses on developing algorithms that enables computers to learn from and make decisions based on data. They rely on specific programming, to identify patterns in data, allowing for improvement over time without manual input (Chen et al.)
Deep Learning
Another specific form of AI is deep learning, which is a type of machine learning that uses neural networks with many layers to analyse complex data. These networks are designed to automatically learn and extract features from raw data, making it possible to detect patterns without needing humans to manually define them (Chen et al.)
The Role of AI in Functional Imaging
The use of AI in conjunction with functional magnetic resonance imaging (fMRI) has proven useful in multiple areas of healthcare. This range spans foetal analysis, segmentation, and psychiatric diagnoses.
Image Quality
Foetal MRI scans have been advanced through the applications of deep learning models. AI contributes to the pre-processing and post-processing stages of functional imaging. Deep learning models are used to detect foetal positioning, ensuring that images are captured from the most optimal angles. This capability reduces the chances of image distortions, leading to more accurate interpretations.
Segmentation
Another application of AI in foetal MRI is the automated brain segmentation process used to separate the parts of the image showing the brain from the rest of the data. Typically, brain segmentation and reconstruction require manual efforts from radiologists, but AI algorithms, particularly convolutional neural networks (CNNs), a form of deep learning, have made this process significantly faster and more accurate (Vahedifard et al.) (Meshaka et al.) An example of AI generated segmentation can be seen in Figure 1.
By automating segmentation, AI reduces human error and enhances the consistency of these critical analyses.
Pregnancy Risk Prediction
AI is also helping predict placental insufficiency in foetal MRI. By using U-Net CNN models (Ronneberger et al.), AI can automatically segment the placenta and generate a prediction score . This model assesses the likelihood of placental problems, allowing for earlier interventions and better management of high-risk pregnancies (Meshaka et al.) . This application of AI not only enhances diagnostic capabilities but also improves the decision-making process for clinicians.
Psychiatric Medicine Applications
Additionally, AI-enhanced fMRI is a developing tool in psychiatric medicine, especially for disorders like schizophrenia. By analysing brain activity patterns, AI models can identify subtle neural changes associated with psychiatric conditions. These advancements help in early diagnosis and understanding the underlying mechanisms of disorders, leading to more precise and personalized treatments. Machine learning techniques, including deep learning, have shown great potential in detecting biomarkers and offering insights into the brain's functional connectivity, improving both diagnosis and treatment planning for patients with psychiatric conditions (Stefano et al.)
Challenges
Although AI has the potential to revolutionize functional imaging by improving accuracy, automating processes, and aiding in clinical diagnoses there are several challenges that must be addressed to fully integrate AI into this field.
Data Quality
A large challenge is ensuring that AI models are trained on high-quality, standardised data. Functional imaging involves complex data that can vary depending on the imaging technique, scanner type, and patient conditions. Without consistent imaging protocols, AI models may produce unreliable results, limiting their effectiveness in clinical applications.
The Radiological Society of North America (RSNA) and its Quantitative Imaging Biomarkers Alliance (QIBA) play a key role in addressing this issue. Their goal is to improve the accuracy and reliability of imaging by standardising biomarkers used to detect and monitor diseases such as cancer ((Bassett)). Standardised imaging data is crucial for training AI models that can be widely applied across different hospitals and imaging centres.
Workflow Integration
For AI to be useful in functional imaging, it must be seamlessly integrated into clinical workflows. This requires collaboration between radiologists, AI developers, and regulatory bodies to ensure that AI tools meet medical standards and can be trusted in real-world applications. The RSNA and QIBA help bridge this gap by working with stakeholders to develop standardised imaging protocols, making it easier to incorporate AI into precision medicine (Bassett).
Accuracy
AI has the potential to enhance precision medicine by improving disease detection, monitoring, and treatment planning. However, without proper validation and standardiszation, AI-driven functional imaging may lead to inconsistent diagnoses (Bassett)) . Ensuring that AI models work reliably across diverse populations and imaging settings remains a significant challenge.
Regulatory Considerations
The use of artificial intelligence (AI) in functional imaging falls under the category of Software as a Medical Device (SaMD)—software that performs medical functions such as diagnosis and analysis. As AI plays a critical role in medical decision-making, strict regulatory oversight is required to ensure safety, reliability, and effectiveness.
International Standards
The International Medical Device Regulators Forum (IMDRF) provides a framework for evaluating SaMD, including AI applications in medical imaging (Larson et al.) . This framework focuses on key areas such as:
Risk assessment – Evaluating risks with AI-driven imaging tools.
Clinical validation – Ensuring AI models are tested on diverse datasets to confirm accuracy.
Continuous performance monitoring – AI systems must be regularly updated and monitored to maintain high standards of safety and reliability.
By following these guidelines, regulators ensure that AI imaging tools meet safety and effectiveness standards prior to clinical use and continue to perform reliably after being introduced to the market.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing AI-based medical devices, including those used in imaging (Ryan et al.)
For example, the FDA introduced the voluntary Software Precertification Program, which aims to simplify the approval process for Software as a Medical Device (SaMD). This program focuses on evaluating the quality systems and practices of manufacturers rather than just the software itself. Companies must show robust systems for quality control and organisational excellence to participate in this program (Ryan et al.)
Continuous Learning and Updates
Furthermore, as AI-driven imaging tools often improve over time, the FDA has proposed a framework that allows for continuous development. To ensure ongoing safety and performance, manufacturers must submit a Predetermined Change Control Plan during the initial review of their software. This plan outlines expected updates to the software and includes a detailed strategy for testing and improving the software post-release, ensuring it remains effective as it evolves (Ryan et al.)
By incorporating these regulatory measures, it enables the continued advancement of AI in functional imaging while safeguarding patient care.
Where to find out more
Romilly Life Sciences can offer several decades experience leading the validation, regulatory approval and implementation of novel technologies including the pivotal development of diagnostic tools and biomarkers based on functional imaging data.
To find out how you can reach patients faster, backed by compelling evidence, contact us.
References
Bassett, Mike. “RSNA’s QIBA: A Quantitative Success.” Rsna.org, 2025, www.rsna.org/news/2021/april/qiba-a-quantitative-success. Accessed 19 Mar. 2025.
Chen, Zhe Sage, et al. “Modern Views of Machine Learning for Precision Psychiatry.” Patterns, vol. 3, no. 11, 11 Nov. 2022, p. 100602, www.sciencedirect.com/science/article/pii/S2666389922002276#abs0010, https://doi.org/10.1016/j.patter.2022.100602. Accessed 26 Feb. 2023.
Larson, David B., et al. “Regulatory Frameworks for Development and Evaluation of Artificial Intelligence–Based Diagnostic Imaging Algorithms: Summary and Recommendations.” Journal of the American College of Radiology, vol. 0, no. 0, 20 Oct. 2020, www.jacr.org/article/S1546-1440(20)31020-6/fulltext, https://doi.org/10.1016/j.jacr.2020.09.060. Accessed 24 Jan. 2021.
Lo, Justin, et al. “Cross Attention Squeeze Excitation Network (CASE-Net) for Whole Body Fetal MRI Segmentation.” Sensors, vol. 21, no. 13, 30 June 2021, p. 4490, https://doi.org/10.3390/s21134490. Accessed 19 Mar. 2025.
Meshaka, Riwa, et al. “Artificial Intelligence Applied to Fetal MRI: A Scoping Review of Current Research.” The British Journal of Radiology, 18 Mar. 2022, https://doi.org/10.1259/bjr.20211205. Accessed 20 May 2022.
Ronneberger, Olaf, et al. “U-Net: Convolutional Networks for Biomedical Image Segmentation.” ArXiv.org, 18 May 2015, arxiv.org/abs/1505.04597.
Ryan, et al. “US FDA Approval of Pediatric Artificial Intelligence and Machine Learning–Enabled Medical Devices.” JAMA Pediatrics, 16 Dec. 2024, https://doi.org/10.1001/jamapediatrics.2024.5437. Accessed 31 Dec. 2024.
Smith, S M. “Overview of FMRI Analysis.” The British Journal of Radiology, vol. 77, no. suppl_2, Dec. 2004, pp. S167–S175, cfn.upenn.edu/stslab/wiki/lib/exe/fetch.php/fmri_club:preprocess1:smith_2004_brjrad.pdf, https://doi.org/10.1259/bjr/33553595.
Stefano, Valeria Di, et al. “Decoding Schizophrenia: How AI-Enhanced FMRI Unlocks New Pathways for Precision Psychiatry.” Brain Sciences, vol. 14, no. 12, 27 Nov. 2024, pp. 1196–1196, www.mdpi.com/2076-3425/14/12/1196, https://doi.org/10.3390/brainsci14121196. Accessed 6 Dec. 2024.
Vahedifard, Farzan, et al. “Artificial Intelligence in Fetal Resting-State Functional MRI Brain Segmentation: A Comparative Analysis of 3D UNet, VNet, and HighRes-Net Models.” ArXiv.org, 2023, arxiv.org/abs/2311.10844. Accessed 19 Mar. 2025.


Comments